Skip to content
Proposed EtO Emissions Regulations
On April 11, 2023, the EPA issued two proposals aimed at reducing ethylene oxide (EtO) emissions, including more stringent air emissions standards and additional protections for workers who are exposed to the gas used to sterilize medical devices and certain spices. If finalized, the EPA’s proposals are estimated to cut EtO emissions from commercial sterilization facilities by 80% per year and apply more protective standards to control those emissions under the law.
“These proposals build on EPA’s extensive outreach to communities across the nation and [reflect] close coordination among key federal partners,” said EPA Administrator Michael S. Regan in an Agency press release. “Together they would significantly reduce worker and community exposure to harmful levels of [EtO]. EPA will continue to use every available tool to safeguard our nation’s communities, including workers, from exposure to toxic chemicals and to deliver important public health protections.” 
CAA proposal
Under the EPA’s Clean Air Act (CAA) authority, the Agency is issuing a proposed rule under the National Emissions Standards for Hazardous Air Pollutants (NESHAP) for Commercial Sterilization Facilities, which are stationary source standards for hazardous air pollutants. The proposed standards outline new requirements for 86 commercial sterilizers across the country. If finalized, the proposal would reduce EtO emissions from these facilities by 80%, bringing emissions levels down so that risk falls below the EPA’s CAA benchmark for elevated cancer risk.
“While many of these facilities have already taken steps to reduce emissions, the proposal will require all 86 facilities and any new facilities to comply with these stricter pollution controls, which have already proven to be effective and achievable,” adds the EPA news release. “All commercial sterilizers will also be required to use advanced source monitoring methods to confirm that these pollution controls are operating effectively. Facilities would be required to report results to EPA twice per year. Under the proposal, facilities would be required to comply with these new requirements within 18 months. This represents an expedited timeline under EPA authority.”
Before drafting the proposed regulation, the EPA required all commercial sterilizers to submit detailed information about EtO emissions and control technologies as part of a 2021 Information Collection Request.
“EPA used this data to estimate risk to people who live near these facilities,” the EPA news release continues. “EPA also conducted extensive pre-proposal outreach in 2022, including community meetings and webinars, which supported state and local efforts to protect communities and generated information that informed and strengthened this proposal.”
New safeguards to protect workers, communities and reduce exposure
The second proposed regulation falls under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA), under which the EPA approves the use of pesticides subject to certain controls through a registration decision, including antimicrobial pesticides like EtO. The new proposed regulation will increase control measures on the use of EtO in the Proposed Interim Decision, such as:
-
Prohibiting certain uses of EtO when alternatives exist, including use in museums, archival settings, beekeeping, some cosmetics, and musical instruments;
-
Reducing the amount of EtO that may be applied for medical device sterilization while meeting applicable standards for sterility assurance;
-
Requiring engineering controls that reduce worker exposures to EtO, such as automation or emissions capture technology; and
-
Mandating personal protective equipment (PPE) in sterilization facilities when EtO is detected using state-of-the-art monitoring technology.
Some commercial sterilization facilities have already successfully implemented some of these measures, including reducing the amount of EtO used for sterilization and installing engineering controls that reduce worker and community exposures. The EPA’s proposal would now require these measures nationwide to further protect workers at EtO commercial sterilization facilities and healthcare facilities and people in communities near these facilities. The proposal includes different timelines for controls depending on their complexity and feasibility. For example, workers can use respirators far more quickly than it takes to reengineer control systems.
The proposal sets forth regulations utilizing real-time monitoring of EtO with technology that can accurately measure EtO within sterilization facilities down to 10 parts per billion (ppb). If levels surpass 10 ppb, workers would be required to wear PPE. The EPA is also instructing industry to develop technologies and methods to identify lower concentrations of EtO—below 10 ppb—inside contract sterilization facilities.
The proposal also includes new data collection and reporting requirements that would help identify and improve protective monitoring technologies and assess the effectiveness of the proposed mitigation measures. Based on this data, the EPA intends to initiate the next round of registration review for EtO earlier than the mandated time frame, including assessing these measures and incorporating additional protections based on advances in technology that occur.
Industries impacted by these proposals include medical device and instrument manufacturing, pharmaceutical preparation, spice and extract purifying, and packaging and labeling services.
Public reactions
In response to the proposed regulations, the American Chemistry Council (ACC) issued the following statement: 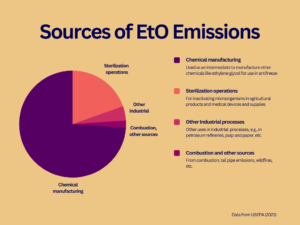
“We support strong, science-based regulations for [EtO]. However, we oppose the use of EPA’s flawed IRIS value as a benchmark in any rulemaking. ACC and others have detailed the severe science-based flaws with the IRIS value that resulted in an overly conservative value that is below background levels of [EtO]. Notably, the Texas Commission on Environmental Quality (TCEQ) identified several flaws underlying EPA’s conclusions, and after conducting its own analysis, TCEQ’s peer-reviewed assessment concluded that the risk of [EtO] is 4,000 times lower than what the IRIS program identified. In fact, the IRIS program’s proposed toxicity value is 19,000 times lower than naturally occurring levels of [EtO] found in the human body. To date, EPA has ignored or failed to provide a meaningful response to the core, scientific, and substantive issues ACC and TCEQ have raised with the development of the IRIS value for [EtO].”
The Earthjustice press release stated the proposed regulations don’t go far enough.
“Today’s proposals are an important first step in remedying an injustice that affects far too many communities,” said Earthjustice Attorney Marvin Brown in the organization’s press release. “Too many workers and community members have gotten cancer from facilities that are supposed to make sure that our medical equipment is safe. We know, and EPA knows, that [EtO] poses a dire cancer risk to anyone who breathes it in. While EPA must move quickly to reduce [EtO] emissions, it must go further and ensure that frontline communities have the data to know when their air is safe through fenceline monitoring. And the agency must move quickly to reduce and phase out the use of [EtO] for sterilizing products that can be safely sterilized by other means.”
For more information or assistance with your Environmental and Health & Safety regulatory compliance needs, contact Ralph Carito at Total Environmental & Safety, LLC (Total) at rcarito@TotalEnviron.com or 908-442-8599.
Thank you for your continued support. If you like what you read in Total’s Monthly EHS Newsletter, please tell your friends and colleagues.
We always appreciate hearing from you, so if you have a suggestion, comment or gripe, please drop us a line at: contact@TotalEnviron.com or call us at 908-442-8599.
Total Environmental & Safety’s (Total’s) most recent blog. Let us know if you enjoyed it.
Page load link




